Evaluation of the efficacy of three medical device detergents on bacteria and yeast derived biofilm: a comparative study
CONTENTS
Original article
Park K, Kim MN, Sung H. Evaluation of the efficacy of three medical device detergents on bacteria and yeast derived biofilm: a comparative study. Ann Clin Microbiol 2023;26:117-24.
Annals of Clinical Microbiology (Ann Clin Microbiol) 2023 December, Volume 26, Issue 4, pages 117-124. https://doi.org/10.5145/ACM.2023.26.4.117
Received on 26 October 2023, Revised on 01 December 2023, Accepted on 01 December 2023, Published on 20 December 2023.
Evaluation of the efficacy of three medical device detergents on bacteria and yeast derived biofilm: a comparative study
Abstract
Background: This study aimed to evaluate the efficacy of three medical detergents against bacteria and yeast-derived biofilms. Methods: The biofilm removal efficacy of EmpowerTM (Metrex, USA), CidezymeTM (Johnson and Johnson Medical Inc, USA), and Matrix mintTM (Whiteley Medical, Australia) were compared to that of chlorine bleach. Biofilms were produced using Staphylococcus aureus RN9120, Escherichia coli ATCC35218, Pseudomonas aeruginosa ATCC27853, Candida albicans ATCC14053, and clinical isolates of Enterococcus faecalis, E. coli, Klebsiella pneumoniae, Candida auris, and Trichosporon asahii. The organisms were suspended in tryptic soy broth (TSB) in 96-well microplates and cultured for 72 hours. They were treated with the detergents, and the residual biofilm mass was quantified using crystal violet staining followed by optical density measurements at 620 nm (OD620 ). Results: EmpowerTM and CidezymeTM significantly reduced the biofilm mass derived from all species by > 50% of OD620 at 37ºC except those from E. faecalis, T. asahii, and C. auris. Matrix mintTM had no effect on the biofilms under any condition. Conclusion: The culture conditions and the species of the biofilm-producing organism influenced the effectiveness of the detergent. Biofilms produced by E. faecalis, C. auris, and T. asahii were resistant to all detergent treatments under all conditions.
Keywords
Biofilm, Detergent, Bacteria, Yeast, Efficacy
Introduction
Materials and methods
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
Study organisms
Measurement of biofilm production
Measurement of biofilm removal efficacy
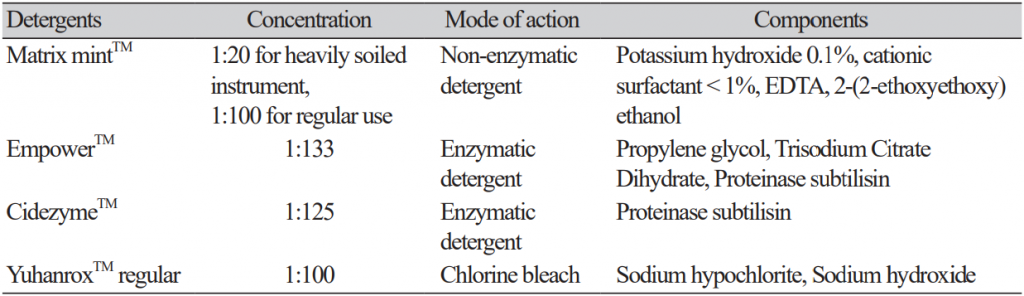
The study detergents included two enzymatic cleaners, EmpowerTM (Metrex, Orange, CA, USA), Cidezyme TM (Johnson and Johnson Medical Inc, Arlington, TX, USA), one non-enzymatic cleaner Matrix mint TM (Whiteley Medical, Sydney, Australia). A chlorine bleach YuhanroxTM Regular (Yuhanclorox, Seoul, Korea) was also tested as a control (Table 1). Each biofilm-producing strain was cultured for 72 hr at the same condition of biofilm production to prepare a 96-well microplate coated with biofilm. The biofilmproducing plates prepared by each strain was tested with each detergent under five different conditions: treatment for 8 min once, two times of 8 min, three times of 8 min, and 30 min once at room temperature (RT) and 30 min once at 37℃. After the detergent treatment, OD620 was measured after elution with 100 μ L 95% ethanol in crystal violet-stained wells. The assay value of OD620 was determined by subtracting the value of blank control cultured with TSB medium only from the original value. All OD620 values smaller than the value of blank control were regarded as 0. A single 8-min treatment with DW at RT was taken as the reference for 0% biofilm removal efficacy.
Statistical analysis
For each condition, the mean and standard deviation of three replicates were assigned to OD620. Using an independent samples t-test (two tailed), biofilm production ability was compared between strains and removal efficacy was compared between detergents. Microsoft Excel (Microsoft Corp., Redmond, WA, USA) was used for statistical analysis.
Results
Biofilm formation of study isolates
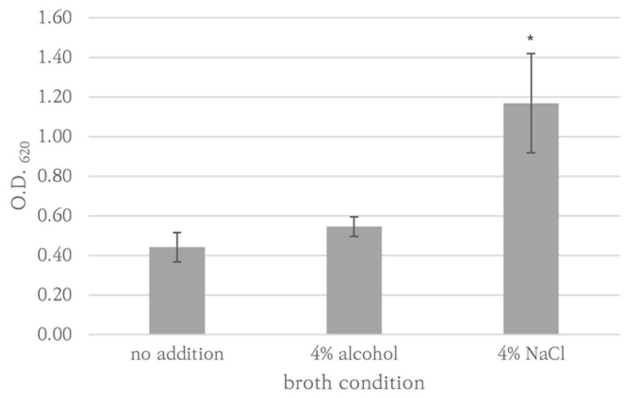
P. aeruginosa ATCC27853, K. pneumoniae AMC244, E. coli ATCC35218, E. coli AMC6615, E. faecalis AMC98901, S. aureus RN9120, C. albicans ATCC14053, C. auris AMC1704, and T. asahii AMC11511 produced significant biofilm production (Fig. 1). S. aureus RN9120 showed an OD620 < 0.5 when cultured with TSB. Because biofilm production of this strain was significantly increased in 4% NaCl-TSB (P = 0.017), but not in 4% ethanol-TSB (P = 0.170) (Fig. 2), it was cultured in 4% NaCl-TSB in biofilm production step when used to test removal efficacy.
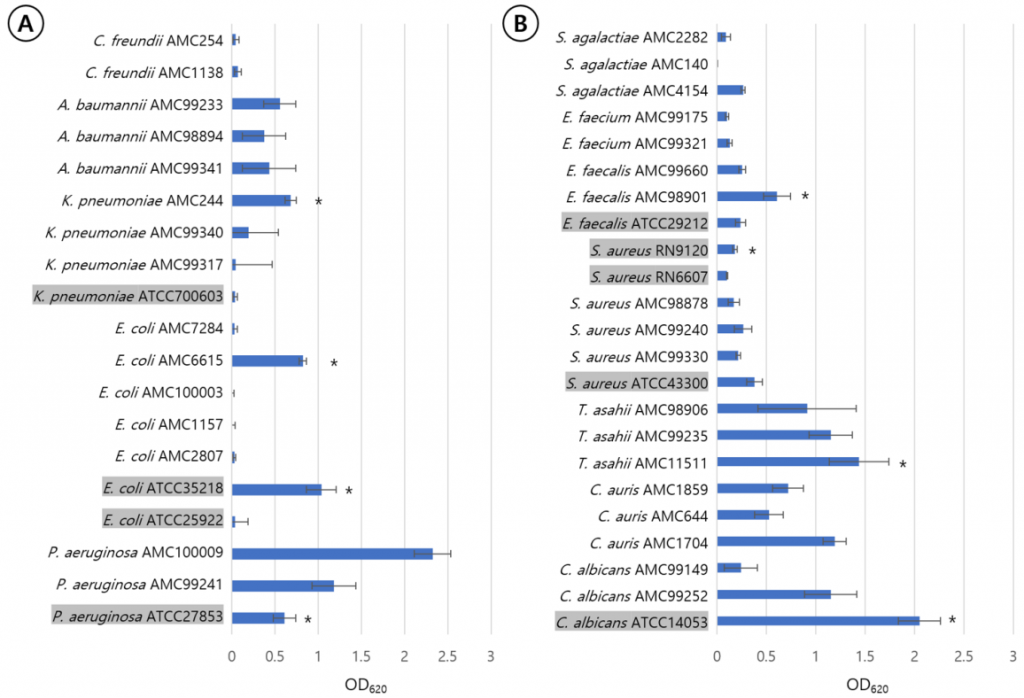
Fig. 1. Quantification of biofilm formation of each strains among gram-negative rods (A) and gram-positive cocci and yeasts (B) using measuring optical density of crystal violet stained biomass at 620 nm. The standard errors are expressed as error bars. The strains marked with asterisks are submitted to the evaluation of biofilm removal. Type strain names were shaded and the strains derived from clinical isolates were given names starting with AMC.
Biofilm removal efficacy of three detergents
Yuhanrox showed consistent biofilm removal efficacy of > 70% at 37°C incubation for all study isolates. Two enzymatic detergents had biofilm removal rates of greater than 50% for biofilm derived from P. aeruginosa ATCC27853, E. coli ATCC35218, E. coli AMC6615, C. albicans ATCC14053, and S. aureus RN9120 at 37°C for 30 min; 66%, 82%, 52%, 82%, and 71% by Cidezyme, and 13%, 80%, 69%, 92%, and 71% by Empower, respectively. For K. pneumoniae AMC244-derived biofilm, Empower showed the removal rates of 42%, 75%, and 55%, respectively, with treatment for 1, 2, and 30 min at RT, while 5% only when 30 min-treatment at 37°C. None of the detergents were effective enough to remove > 30% of biofilms derived from E. faecalis AMC98901, C. auris AMC1704, and T. asahii AMC11511. Matrix did not show significant removal efficacy against biofilm under any strain or condition (Fig. 3).
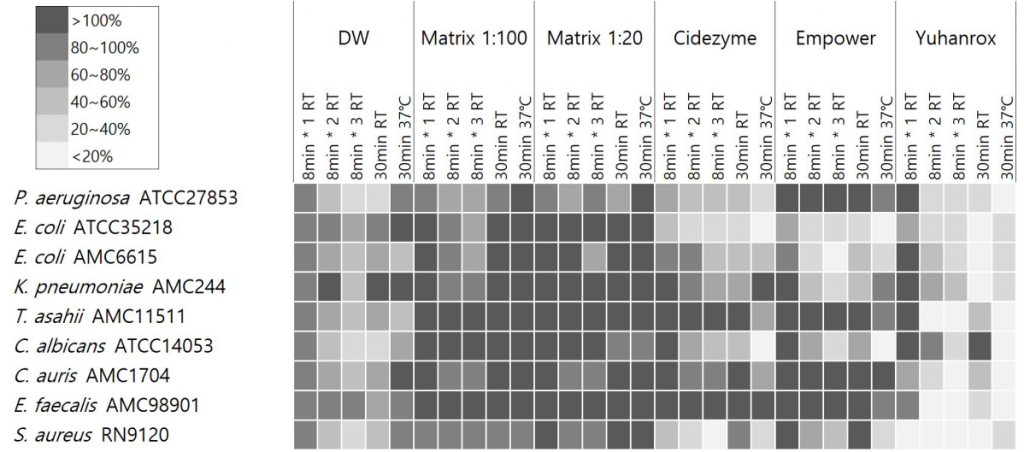
Fig. 3. Residual biofilm percentage after treatment of study detergents. The percentage OD620 after treatment divided by baseline OD620 before detergent treatment was expressed in gray scale with shaded box for the corresponding range. The shade and biofilm removal of each well were inversely correlated. The study detergents included two enzymatic cleaners EmpowerTM (Metrex, Orange, CA), CidezymeTM (Johnson and Johnson Medical Inc, Arlington, TX), one non-enzymatic cleaner Matrix mintTM (Whiteley Medical, Sydney, Australia). A chlorine bleach YuhanroxTM Regular (Yuhanclorox, Seoul, Korea) was also tested as a control. Abbreviations: DW, distilled water; RT, room temperature.
Discussion
References
1. Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003;2:114-22.
2. Sutherland IW. The biofilm matrix-an immobilized but dynamic microbial environment. Trends Microbiol 2001;9:222-7.
3. Flemming HC and Wingender J. Relevance of microbial extracellular polymeric substances (EPSs)–part I: structural and ecological aspects. Water Sci Technol 2001;43:1-8.
4. Centers for Disease Control and Prevention. Guideline for disinfection and sterilization in healthcare facilities (2008). https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ index.html [Online] (last visited on 1 Septebmer 2023).
5. Wang S, Zhao Y, Breslawec AP, Liang T, Deng Z, Kuperman LL, et al. Strategy to combat biofilms: a focus on biofilm dispersal enzymes. NPJ Biofilms Microbiomes 2023;9:63.
6. Wilcox MH, Fawley WN, Wigglesworth N, Parnell P, Verity P, Freeman J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium dif f icile infection. J Hosp Infect 2003;54:109-14.
7. Gonzalez JA, Vanzieleghem T, Dumazy A, Meuris C, Mutsers J, Christiaens G, et al. On-site comparison of an enzymatic detergent and a non-enzymatic detergent-disinfectant for routine manual cleaning of flexible endoscopes. Endosc Int Open 2019;7:E412-20.
8. Zühlsdorf B, Emmrich M, Floss H, Martiny H. Cleaning efficacy of nine different cleaners in a washer–disinfector designed for flexible endoscopes. J Hosp Infect 2002;52:206-11.
9. Fang Y, Shen Z, Li L, Cao Y, Gu LY, Gu Q, et al. A study of the efficacy of bacterial biofilm cleanout for gastrointestinal endoscopes. World J Gastroenterol 2010;16:1019-24.
10. Ren W, Sheng X, Huang X, Zhi F, Cai W. Evaluation of detergents and contact time on biofilm removal from flexible endoscopes. Am J Infect Control 2013;41:e89-92.
11. Vickery K, Pajkos A, Cossart Y. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am J Infect Control 2004;32:170-6.
12. da Costa Luciano C, Olson N, Tipple AF, Alfa M. Evaluation of the ability of different detergents and disinfectants to remove and kill organisms in traditional biofilm. Am J Infect Control 2016;44:e243-9.
13. Sakoulas G, Eliopoulos GM, Moellering RC, Jr., Wennersten C, Venkataraman L, Novick RP, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother 2002;46:1492-502.
14. Mangwani N, Shukla SK, Kumari S, Das S, Rao TS. Effect of biofilm parameters and extracellular polymeric substance composition on polycyclic aromatic hydrocarbon degradation. RSC Adv 2016;6:S7540-51.
15. Musleh RM and Jebur AQ. Crystal violet binding assay for assessment of biofilm formation by Klebsiella pneumoniae on catheter, glass and stainless-steel surfaces. Iraqi J Sci 2014;55:120812.
16. Stiefel P, Mauerhofer S, Schneider J, Maniura-Weber K, Rosenberg U, Ren Q. Enzymes enhance biofilm removal efficiency of cleaners. Antimicrob Agents Chemother 2016;60:364752.
17. Stiefel P, Rosenberg U, Schneider J, Mauerhofer S, Maniura-Weber K, Ren Q. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl Microbiol Biotechnol 2016;100:4135-45.
18. Lee S, Choi KH, Yoon Y. Effect of NaCl on biofilm formation of the isolate from Staphylococcus aureus outbreak linked to ham. Korean J Food Sci Anim Resour 2014;34:25761.
19. Borucki MK, Peppin JD, White D, Loge F, Call DR. Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol 2003;69:7336-42.
20. Lianou A, Nychas GE, Koutsoumanis KP. Strain variability in biofilm formation: a food safety and quality perspective. Food Res Int 2020;137:109424.
21. Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015;21:S1-25.
22. Fanning S and Mitchell AP. Fungal biofilms. PLoS Pathog 2012;8:e1002585.
23. Rahman MR, Perisetti A, Coman R, Bansal P, Chhabra R, Goyal H. Duodenoscope-associated infections: update on an emerging problem. Dig Dis Sci 2019;64:1409-18.
24. Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, Baud O, et al. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy 2010;42:895-9.
25. Molobela IP, Cloete TE, Beukes M. Protease and amylase enzymes for biofilm removal and degradation of extracellular polymeric substances (EPS) produced by Pseudomonas fluorescens bacteria. Afr J Microbiol Res 2010;4:1515-24.
26. Tristezza M, Lourenço A, Barata A, Brito L, Malfeito-Ferreira M, Loureiro V. Susceptibility of wine spoilage yeasts and bacteria in the planktonic state and in biofilms to disinfectants. Ann Microbiol 2010;60:549-56.
27. Sun W, Wang Y, Zhang W, Ying H, Wang P. Novel surfactant peptide for removal of biofilms. Colloids Surf B Biointerfaces 2018;172:180-6.
28. Ribeiro MM, Graziano KU, Olson N, França R, Alfa MJ. The polytetrafluoroethylene (PTFE) channel model of cyclic-buildup biofilm and traditional biofilm: the impact of friction, and detergent on cleaning and subsequent high-level disinfection. Infect Control Hosp Epidemiol 2020;41:172-80.
29. Beilenhoff U, Biering H, Blum R, Brljak J, Cimbro M, Dumonceau JM, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – update 2018. Endoscopy 2018;50:1205-34.
30. Maillard JY and Centeleghe I. How biofilm changes our understanding of cleaning and disinfection. Antimicrob Resist Infect Control 2023;12:95.
31. Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect 2013;19:107-12.
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Funding
None.